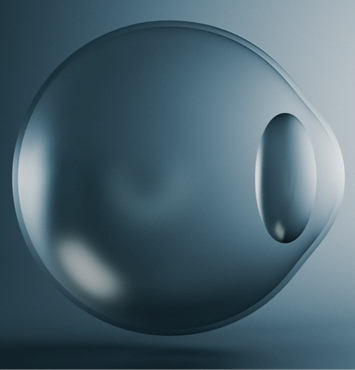
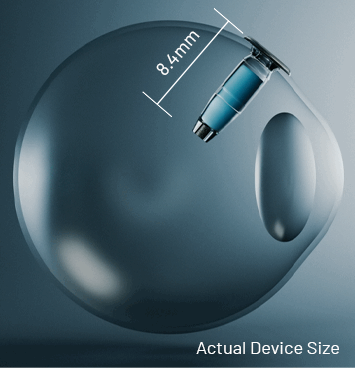
Continuous Delivery of wet AMD Treatment with SUSVIMO®
SUSVIMO is the first and only device for people with wet AMD that continuously delivers treatment.

Over the next 6 months, SUSVIMO slowly releases medicine into your eye, continuously delivering treatment.*

The medicine inside the implant is called ranibizumab. It has been trusted by Retina Specialists for years to treat wet AMD.
There is more to know about the implant insertion and after refill procedure, refer to the SUSVIMO Implant Procedure for more information and procedure guidance.
The SUSVIMO implant is not visible to others, as the eyelid lies naturally over it.
Drag the slider to see the real size of SUSVIMO implant compared to the eye.
“My experience with the implant has been quite smooth. Outside of receiving my refills, I wouldn’t even know it’s there.”
Bill, SUSVIMO Patient
“My experience with the implant has been quite smooth. Outside of receiving my refills, I wouldn’t even know it’s there.”
Bill, SUSVIMO Patient
Patient experiences may vary.
SUSVIMO helps you maintain vision with just 2 treatments per year*†
Go up to 6 months between treatments with SUSVIMO

In clinical trials, patients receiving SUSVIMO experienced a serious eye condition called endophthalmitis at a higher rate than those on monthly ranibizumab injections.
In certain cases, you may be given an additional treatment in the SUSVIMO-treated eye if your Retina Specialist decides it is necessary.
†In a clinical trial, SUSVIMO patients maintained vision as well as patients on monthly ranibizumab injections at weeks 36 and 40, as measured by the best corrected visual acuity score. There was a temporary decrease in vision after the SUSVIMO procedure.
-
-
SUSVIMO [package insert]. South San Francisco, CA: Genentech, Inc; 2022.
SUSVIMO [package insert]. South San Francisco, CA: Genentech, Inc; 2022.
-
Campochiaro PA, Marcus DM, Awh CC, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2019;126:1141-1154.
Campochiaro PA, Marcus DM, Awh CC, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2019;126:1141-1154.
-
SUSVIMO Initial Fill and Implant Procedure Instructions for Use. Genentech, Inc. 2022.
SUSVIMO Initial Fill and Implant Procedure Instructions for Use. Genentech, Inc. 2022.
-
Monés J, Gune S, Maia M, et al. Pharmacokinetic profile of the port delivery system with ranibizumab in the phase 3 Archway trial. Presented at 21st EURETINA Congress, September 9-12, 2021.
Monés J, Gune S, Maia M, et al. Pharmacokinetic profile of the port delivery system with ranibizumab in the phase 3 Archway trial. Presented at 21st EURETINA Congress, September 9-12, 2021.
-
Ranade SV, Wieland MR, Tam T, et al. The port delivery system with ranibizumab: a new paradigm for long-acting retinal drug delivery. Drug Delivery. 2022;29(1):1326-1334.
Ranade SV, Wieland MR, Tam T, et al. The port delivery system with ranibizumab: a new paradigm for long-acting retinal drug delivery. Drug Delivery. 2022;29(1):1326-1334.
-
Data on file. Genentech, Inc. 2021.
Data on file. Genentech, Inc. 2021.
-
Data on file. Genentech, Inc. 2021.
Data on file. Genentech, Inc. 2021.
-
Holekamp NM, Campochiaro PA, Chang MA, et al; Archway Investigators. Archway randomized phase 3 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2022;129(3):295-307.
Holekamp NM, Campochiaro PA, Chang MA, et al; Archway Investigators. Archway randomized phase 3 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2022;129(3):295-307.
-
A phase III study to evaluate the port delivery system with ranibizumab compared with monthly ranibizumab injections in participants with wet age-related macular degeneration. ClinicalTrials.gov identifier: NCT03677934. Updated July 22, 2021. https://clinicaltrials.gov/ct2/show/NCT03677934
A phase III study to evaluate the port delivery system with ranibizumab compared with monthly ranibizumab injections in participants with wet age-related macular degeneration. ClinicalTrials.gov identifier: NCT03677934. Updated July 22, 2021. https://clinicaltrials.gov/ct2/show/NCT03677934
-
Wykoff CC; Archway Investigators. 2-year outcomes from the phase 3 Archway trial: management of neovascular age-related macular degeneration using the port delivery system with ranibizumab (PDS). Presented at Bascom Palmer Eye Institute Angiogenesis, Exudation, and Degeneration 2022 – Virtual Edition, February 11-12, 2022.
Wykoff CC; Archway Investigators. 2-year outcomes from the phase 3 Archway trial: management of neovascular age-related macular degeneration using the port delivery system with ranibizumab (PDS). Presented at Bascom Palmer Eye Institute Angiogenesis, Exudation, and Degeneration 2022 – Virtual Edition, February 11-12, 2022.
-
Data on file. Genentech, Inc. 2021.
Data on file. Genentech, Inc. 2021.
-
Data on file. Genentech, Inc. 2021.
Data on file. Genentech, Inc. 2021.
-
Chang MA, Kapre A, Kaufman D, et al. Patient preference and treatment satisfaction with a port delivery system for ranibizumab vs intravitreal injections in patients with neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmology. Published online June 16, 2022.
Chang MA, Kapre A, Kaufman D, et al. Patient preference and treatment satisfaction with a port delivery system for ranibizumab vs intravitreal injections in patients with neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmology. Published online June 16, 2022.
-
-